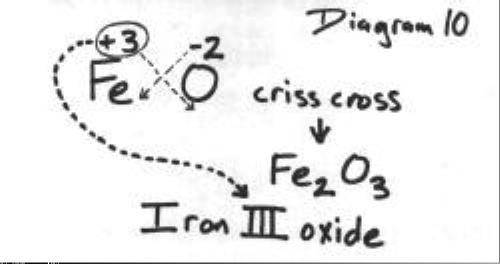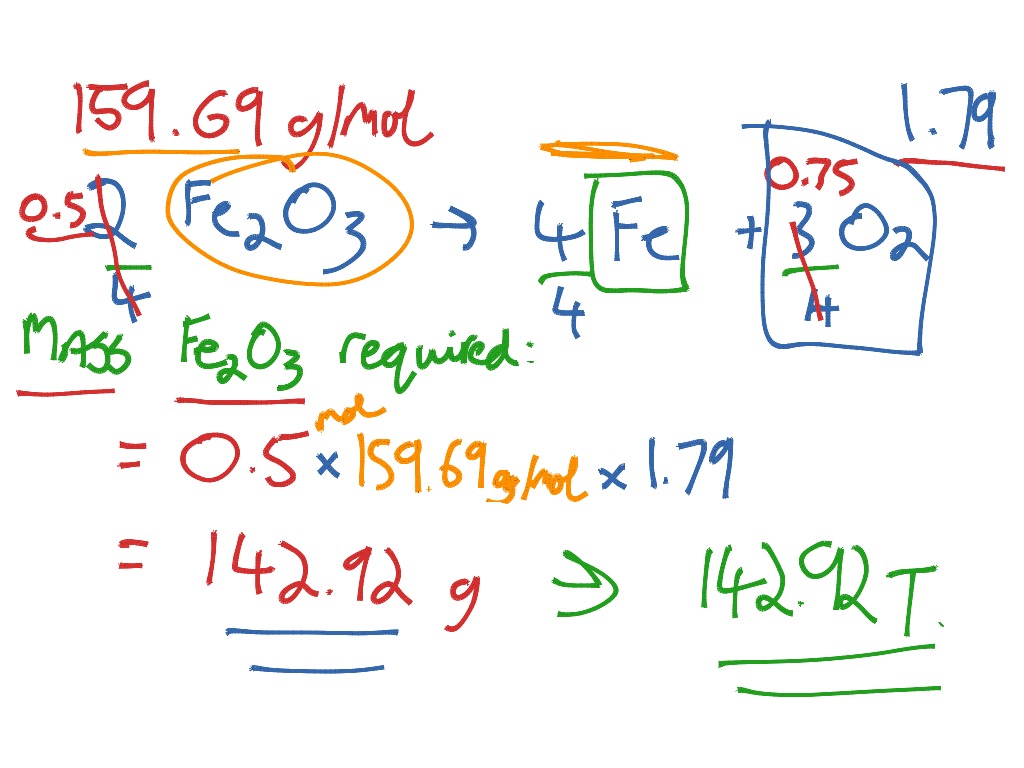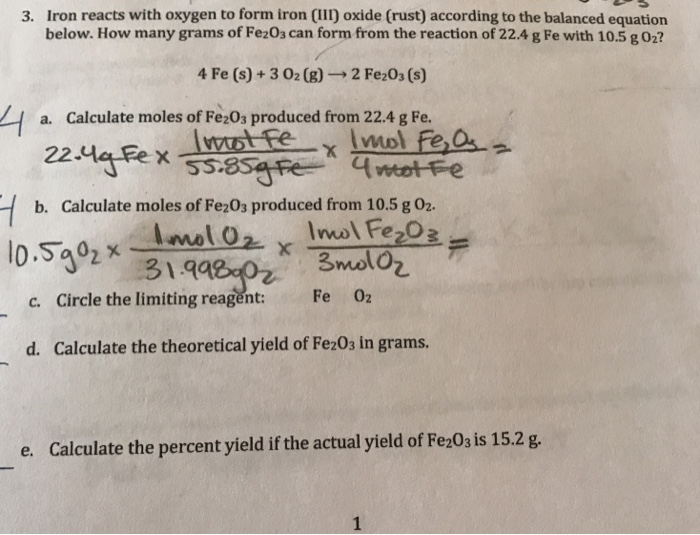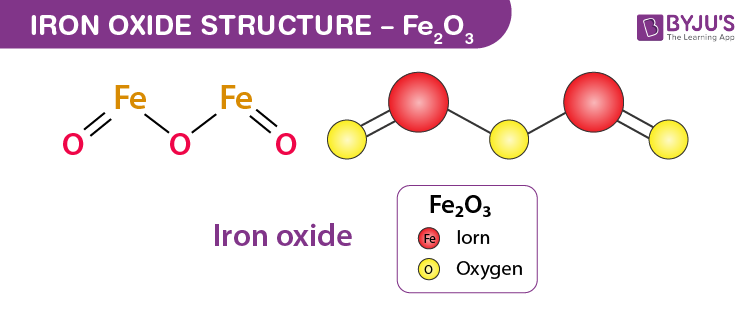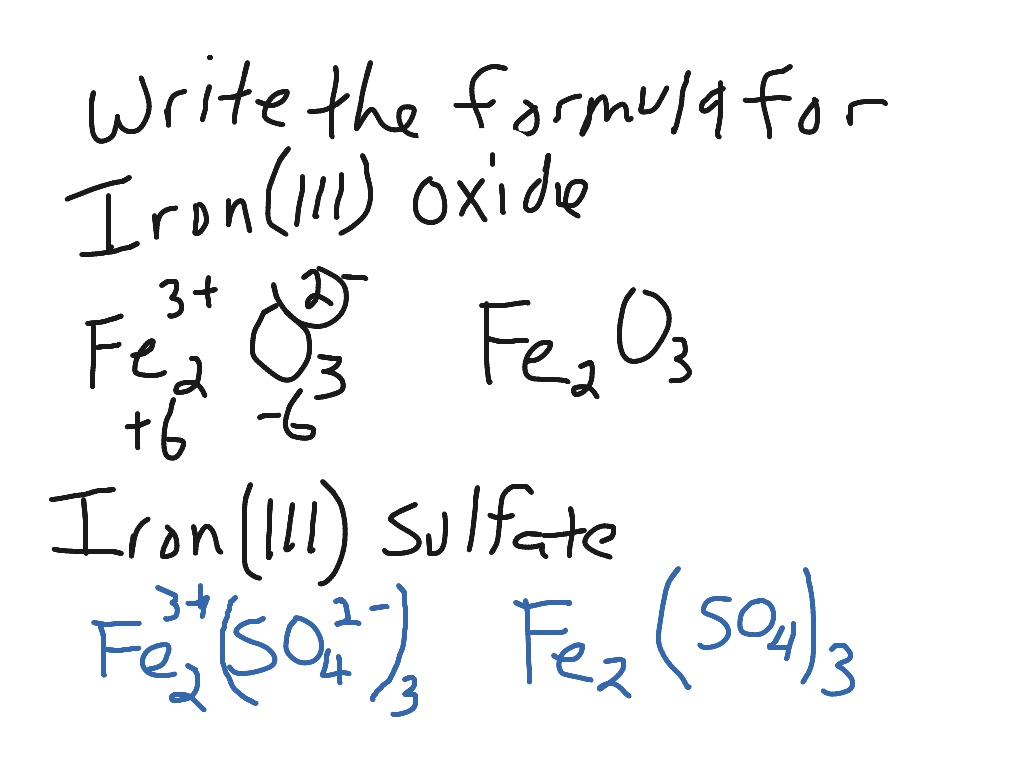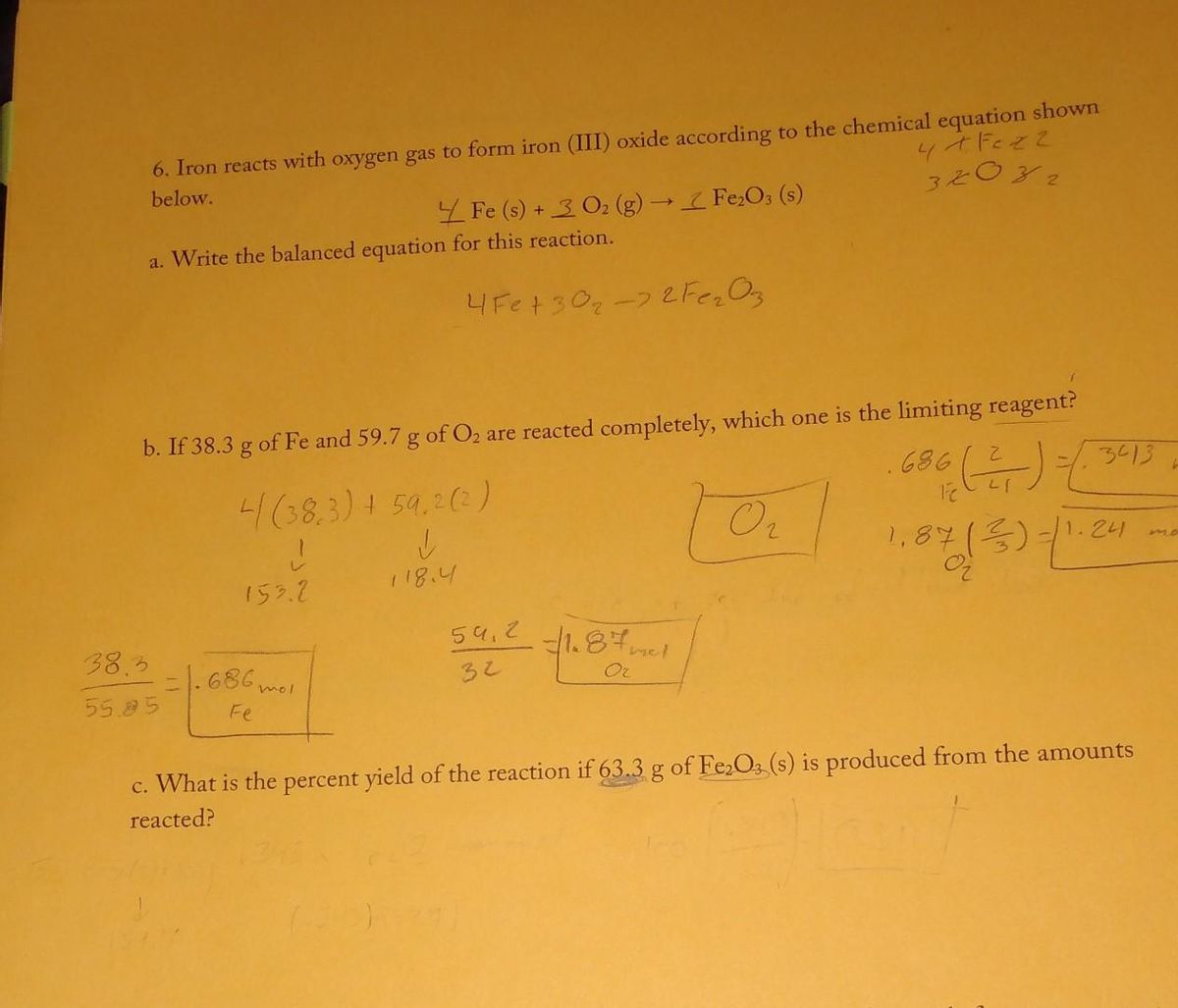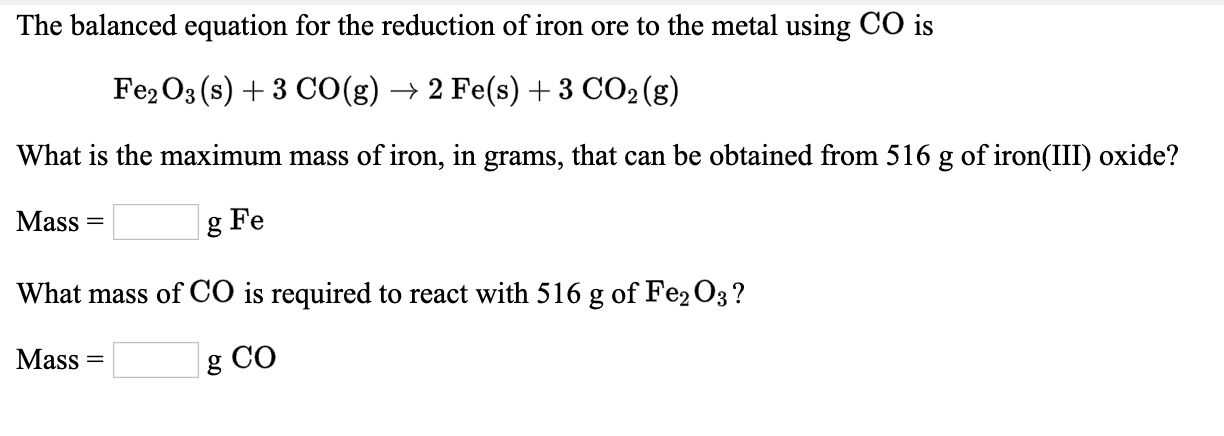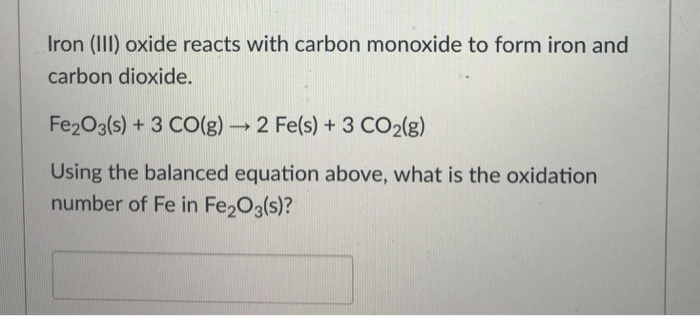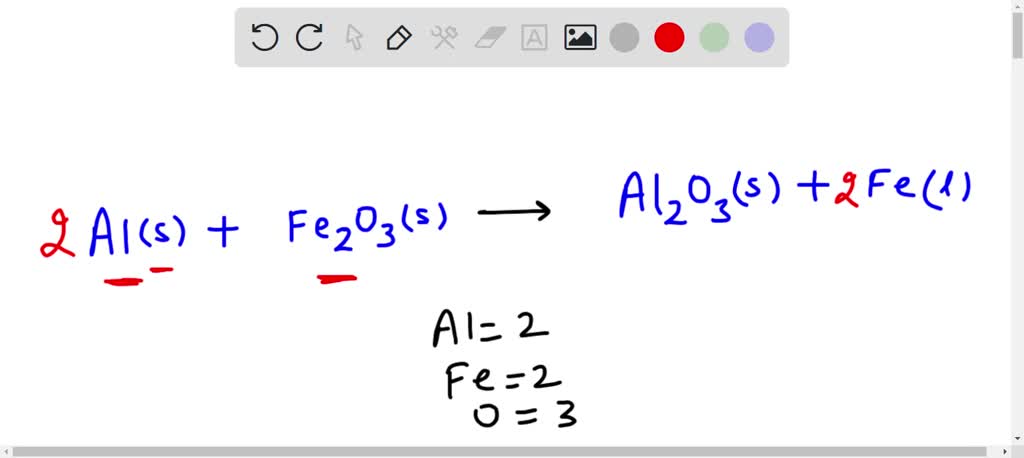
SOLVED: Write a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metal
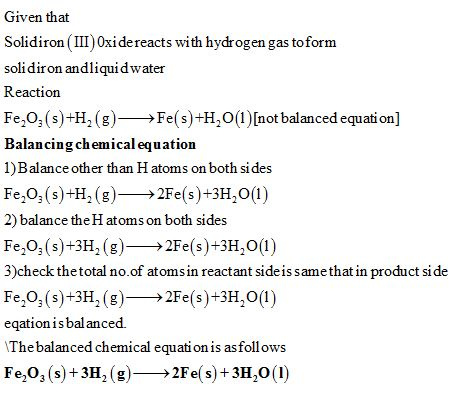
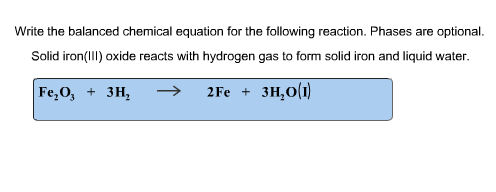
Chemical equation for the following reaction. Phases are optional. Solid iron(III) oxide reacts with hydrogen - Home Work Help - Learn CBSE Forum

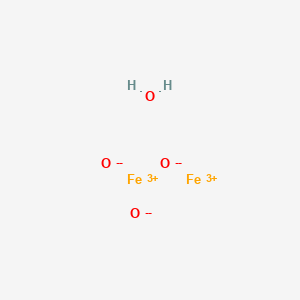
Question Video: Deducing the Ionic Formula of an Ionic Compound Where Both Ions Have Greater-Than-One Charge | Nagwa